Globale UDI-
Anforderungen
schnell und einfach
umsetzen
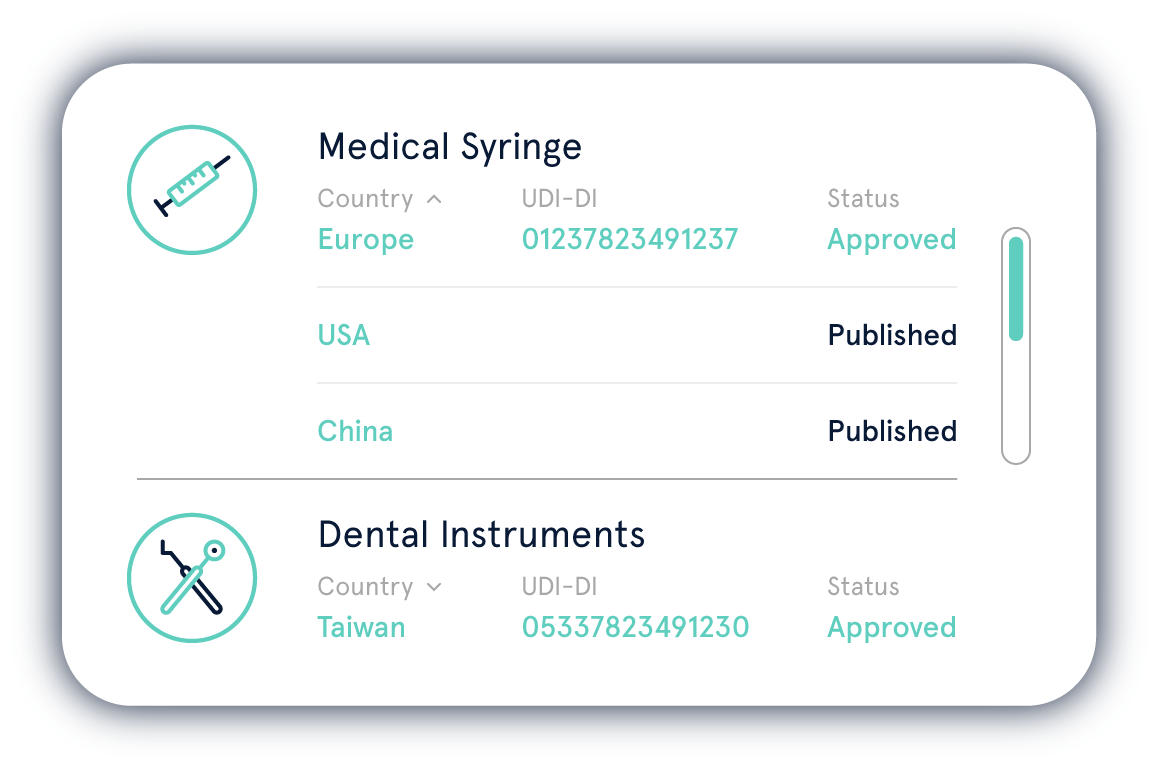
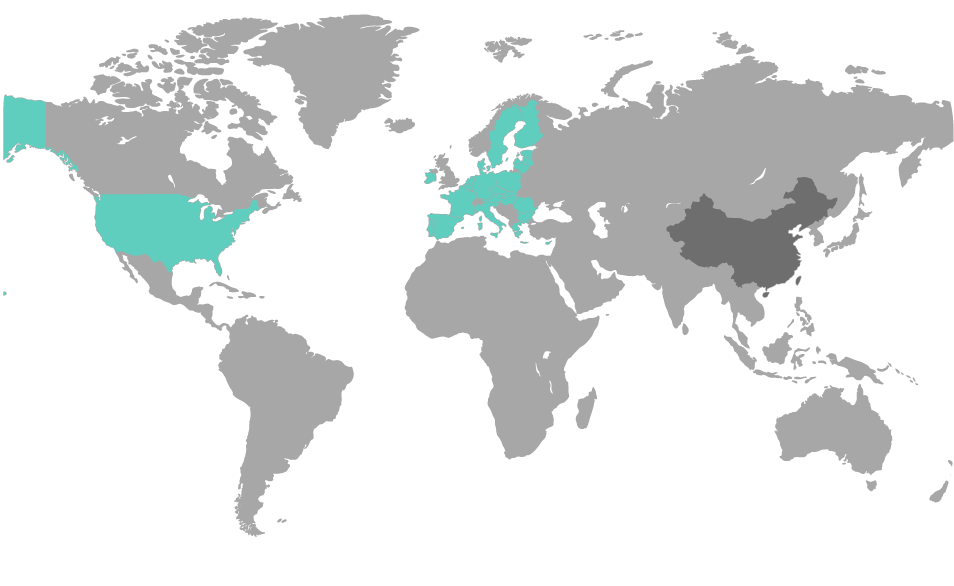
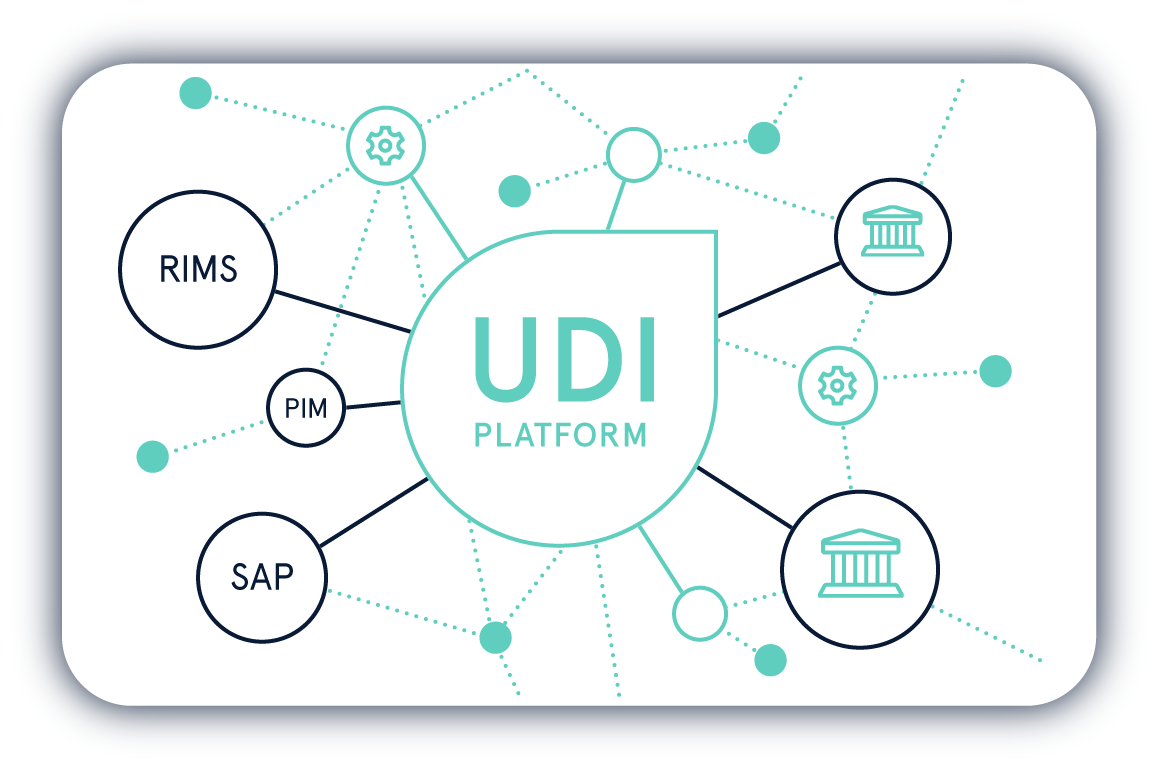
Die Einhaltung der UDI-Regularien von verschiedenen Regulierungsbehörden ist eine komplexe Aufgabe. Mit der UDI Platform behalten Sie Ihre UDI-Daten jederzeit im Griff.